About the research
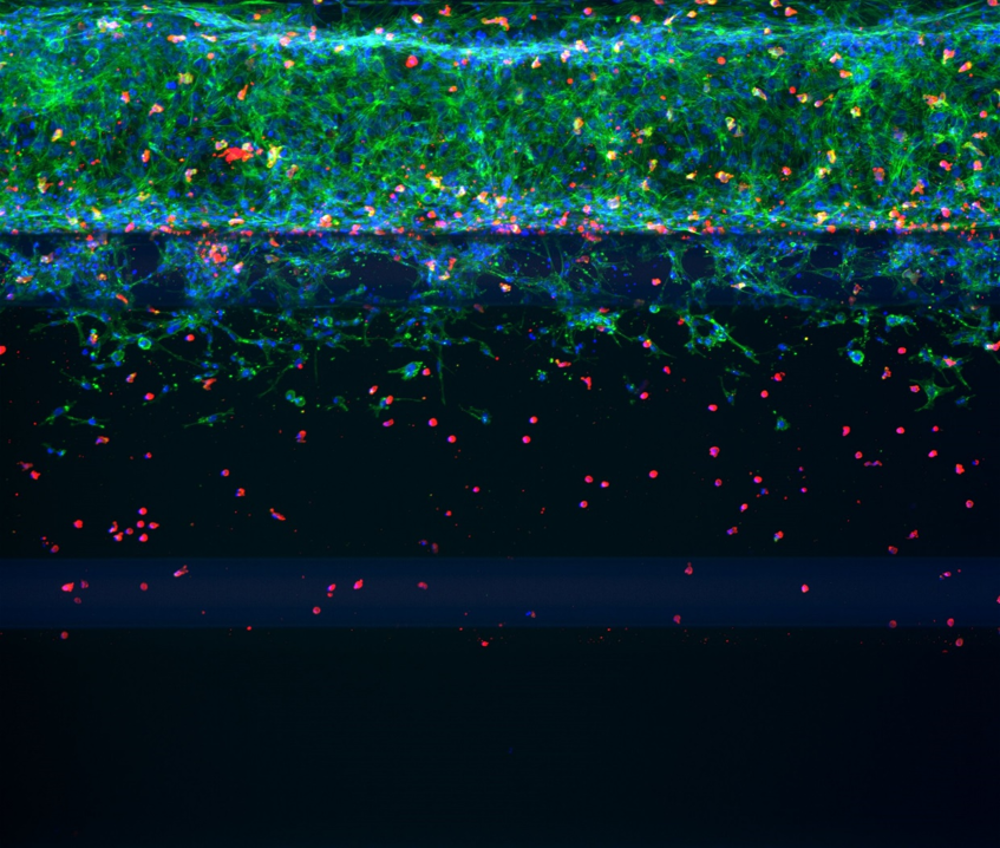
Recruitment and transendothelial migration of T cells is a crucial aspect of immune system functioning and can result in the onset or propagation of disease if aberrant. Most research studying transendothelial migration of T cells is performed in mouse models or in vitro using migration assays across monolayers of cells that do not recapitulate the complex human in vivo situation. For this reason, we developed an endothelial vessel perfused with T cells in a membrane-free and tubular manner against a collagen hydrogel. Tumor cells or chemokines can be added as a chemotactic trigger. Such 3D co-culture models allow studying recruitment and infiltration of T cells in healthy or diseased environments and could support the development of novel (immuno)therapeutics.
The use of a patients’ own immune system proved to be a promising avenue for combatting cancer although therapeutic efficacy varies significantly depending on the tumor type and between patients. Novel models that manage to better recapitulate the behavior of T cells in vivo could potentially spur the development and improvement of these immune therapies. This knowledge led us to the development of a 3D tissue culture model where we could better understand this behavior. The model compromises an endothelial vessel perfused with T cells that can be used to study the migration of the T cells towards a tumor compartment in a high-throughput manner.
Custom model & assay
We have used the OrganoPlate® 3-lane 40 to grow blood vessels perfused with T cells. This type of OrganoPlate features 40 independent tissue culture chips with 3 adjacent channels per chip. We added Collagen I extracellular matrix (ECM) gel to the middle channel and seeded endothelial cells against the ECM gel in the top channel. After adhesion of the endothelial cells, we started perfusion of the culture to form an endothelial tubular structure. Perfusion was performed by placing the OrganoPlate on the OrganoFlow®.
Following T cell migration over time
To eventually mimic the tumor microenvironment, we seeded tumor cells in or against the gel in the bottom perfusion channel. As a final step, we perfused the endothelial vessel with fluorescently stained T cells to follow the migration of T cells towards the tumor compartment for 48 hours. By following this migration over time, we observed that both unstimulated and stimulated T cells migrated towards the tumor cells.
The migration of T cells could be stimulated by adding an additional chemotactic trigger (chemokine) to the medium in the bottom channel or inhibited by pre-treatment of the endothelial tube with a compound.
Key results
- 3D immune cell migration through an extracellular matrix towards a tumor compartment
- T cell extravasation from a blood vessel
- Allows excellent imaging, cell tracking & quantification
- Study the effects of compounds on immune cell migration
Want to know more?
Get up to speed with 3D tissue culture and learn how OrganoPlate® supports your research needs.
