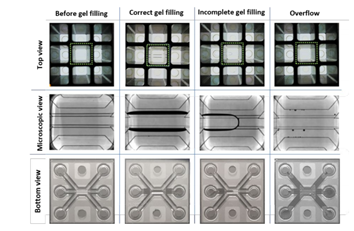
Why does my gel not fill the channel all the way to the end?
Improper filling of the gel channel can occur if you are working with a very viscous gel or if your gel is already partly polymerized. When working with Matrigel® or Matrigel®-like gels, thaw the gel according to the manufacturer’s instructions and make sure the gel is kept on ice continuously to prevent it from polymerizing. Using pre-cooled Eppendorf tips will also prevent this.
If your gel does not fill the gel channel properly, try increasing the seeding volume. For example, for OrganoPlate® 2-lane and 3-lane with a 400µm channel width, the range of collagen-I gel volume to seed varies between 1.5-2.1 µL. We advise starting with a seeding volume of 2 µL. If the gel does not properly fill the channel all the way, increase the seeding volume until you find the volume that works for your experiment. The Sartorius eLINE electronic repeating pipette (#735021) is very well suited for this purpose.