Dissatisfied With Your Angiogenesis Model? Read This
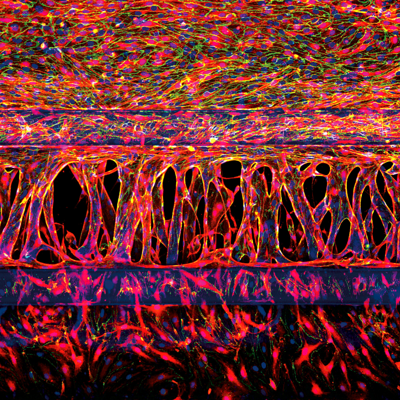
Physiological relevance and logistical aspects of a range of modern angiogenesis in vitro platformsFollowing the recognition that controlling angiogenesis could have therapeutic value, unraveling its mechanisms has become of significant interest.1 Angiogenesis, the formation, and remodeling of new blood vessels and capillaries from the growth of pre-existing blood vessels is a significant contributor to tumor growth, and anti-angiogenic agents have been pursued as a promising approach for some cancers.Understanding the molecular and cellular mechanisms of angiogenesis is also expected to provide insights that lead to improved treatment options for other conditions.2 To achieve clinical success in this area, researchers need robust in vitro models that are specifically designed to study angiogenesis in a defined and well-controlled environment. Conventional in vitro platforms are insufficient for true studies of angiogenesis and better screening and disease models are needed.In this article, we explore the physiological relevance and logistical aspects of a range of modern angiogenesis in vitro platforms, including MIMETAS’ own OrganoPlate®.Why better angiogenesis models are neededTwo-dimensional in vitro assays are often used to study cell migration, a single key aspect of angiogenesis. However, these platforms (such as the scratch assay and Boyden chamber) are incomplete models of angiogenesis that do not feature any vessel formation, so alternative models have been sought.Several versions of the “tube formation assay” have been widely used to measure the ability of endothelial cells to form capillary-like structures known as “tubes”. The main limitations of tube formation assays, which have been used to assess the effects of angiogenic regulators, are described in more detail in our recent blog post “Can You Really Model Angiogenesis In Vitro?”. Overall, tube formation assays and other in vitro alternatives fail to represent several key features of angiogenesis, including:Pre-existing vessels; a necessary component for angiogenesis modeling. Assays that facilitate de novo vessel formation are better representations of vasculogenesis.The formation of endothelial cell lumen. In vivo, this occurs concomitantly with the invasion of vascular sprouts and allows blood to flow through the capillary. This perfusion is essential for capillary function, as well as the maintenance and regulation of lumen diameter3Sprouting cues that regulate vessel growth and remodeling. In addition to perfusion, the presence of a biochemical gradient regulates capillary formation in vivo. Incorporating a reproducible and sustained gradient in an in vitro model is needed to better reflect true angiogenic processes.The need for more complex models is even greater now that there is such a strong interest in finding drugs that target the microvasculature, and high-throughput methods for drug screening are in high demand.Modern in vitro strategies for angiogenesis research: progress and limitationsMicrofluidic devicesThe field of microfluidics has made its way from the microelectronics industry to angiogenesis research, bringing with it the opportunity to more faithfully mimic tissue architecture.4,5 Microfluidic systems allow spatial control over fluids in micrometer-sized channels, and they represent an important step towards acknowledging the complexity of the cellular microenvironment and its significant bearing on physiological systems. Critical cues can be mimicked with varying degrees of success in different microfluidic models including lumen perfusion and the resulting shear stress, and biochemical gradients.6,7 The value of incorporating these factors into angiogenesis models is reflected in a review of 87 papers containing the keywords ‘[microfluidic OR microengineered] and 3D cell culture’ in 2015 that identified the vasculature as the most modeled tissue.4Microfluidic devices are promising tools that are expected to play a significant role in drug discovery, however, they are still largely an emerging technique under development.5 A review of organ-on-a-chip efforts concluded that very few microfluidic systems have managed to incorporate all elements that result in a user-friendly process that is suited to answering biological questions.8 Many microfluidic models need to be manufactured manually before use, which often involves the use of tubing and pumps to supply flow and maintain gradients.9 This requirement increases the complexity of use and limits routine adoption.9 Further effects on scalability and standardization are compounded if there is some incompatibility with imaging tools or other equipment.8Ready-to-use 3D HUVEC cultures in plate insertsVarious three-dimensional tissue culture platforms have been developed to meet the need for angiogenic models that provide pre-existing vasculature growth. Ready-to-use models can be purchased with fluorescently labeled human umbilical vein endothelial cells (HUVECs) that are already seeded in a plate insert, co-cultured with supporting cells that are labeled in a different color. Tubule formation can be monitored in real-time to assess the effect of various compounds on different stages of vascular formation, using fluorescence microscopy. This approach offers significant flexibility, enabling studies of vasculogenesis through advanced angiogenesis. As cells are seeded on a matrix, their invasion through the matrix can be assessed.Challenges lie in maintaining the concentration gradient of angiogenic compounds required for growth, which will dissipate over time and limit the length of experiments. Vessels grown in a three-dimensional matrix within a plate insert can be difficult to access in a way that enables lumen perfusion, an important test used to ascertain whether vessels are functional and prevent leakage.10 HUVEC cultures in plate inserts are also usually not suited to automated imaging, making it difficult to scale up and increase throughput. Some models also require weeks for vascular network formation, as well as high reagent volumes that add to the often-high cost of these inserts.A specialized and high-throughput in vitro microfluidic culture plate for angiogenesis researchTo address the need for high-throughput in vitro platforms that integrate both perfusion and the generation of stable biomolecular gradients, MIMETAS has employed their own tissue culture plate, the OrganoPlate, to establish an angiogenesis model. This gradient-driven, three-dimensional angiogenesis assay provides a standardized format based on a 384-well plate setup and can consist out of 40 or 64 independent chips.4The OrganoPlate® 3-lane 40 comprises 40 tissue culture chips, each consisting of three channels:Top channel: HUVECs are grown in the top channel and form an endothelial vessel under perfusionMiddle channel: Extracellular matrix gelBottom channel: A cocktail of angiogenic factors which induce the directed formation of angiogenic sproutsThe OrganoPlate® 3-lane 64 has similar properties, but comprises 64 tissue culture chips, as well as being specifically optimized for automated workflows, such as automated liquid handling instruments.The channels are patterned by small ridges, known as PhaseGuides, which function as capillary pressure barriers and enable patterning of the cells and gel.4,11 Each of the microfluidic chips is individually addressable; the lumen can be accessed through the perfusion channel, while the gel forms the basal side of the tube.4,12This model is uniquely suited to perform physiologically relevant studies on the formation and regression of the microvasculature in vitro and is compatible with high content imaging equipment. In contrast to other models where it is difficult to apply a stable gradient of growth factors that lasts longer than a few minutes or hours, a stable gradient can be maintained in the OrganoPlate for multiple days.6 This assay lends itself to both scalability and reproducibility; as continuous perfusion is achieved by placing the OrganoPlate on an interval rocker platform set at eight-minute intervals, multiple experiments can be performed at once by stacking culture platforms on top of each other. While the absence of tubing and pumps improves usability and scalability, the reliance on the rocker as a source of flow results in several drawbacks. Unlike in vivo settings, flow within the vessels is bi-directional. As continuous perfusion is required to maintain the vessels and gradient, time-lapse imaging must be obtained at either discrete-time points6 or while the platform is on the rocker.By integrating a biochemical gradient and an accessible and perfusable lumen, the OrganoPlate provides improved physiological relevance that is not offered in many other models. The defined geometry of the microfluidic channels allows many factors to be kept constant across different experiments, including the position and density of the cells, amount of flow, position of the extracellular matrix, and the shape of the gradient.6 Lastly, the platform could be expanded to comprise other cell types, creating further opportunities to ask a wide variety of research questions.6Choosing an in vitro angiogenesis modelWhen choosing an in vitro angiogenesis model, it is important to carefully consider the research question you are asking and to be aware of the benefits and limitations of each model. Are you studying angiogenesis or vasculogenesis? Over what period do you need your gradient or model to be viable? How important is it that you have lumen formation and access? Can the model be adapted to support studies of tumor angiogenesis or other specific mechanisms or diseases?The OrganoPlate has been employed to provide the most complete in vitro angiogenesis assay and offers unique opportunities to study fundamental aspects and regulators of endothelial cell biology. Unlike many other models, the OrganoPlate incorporates the key features of angiogenesis in one assay, while offering a standardized platform suited to studies on a high-throughput scale.6If you want to learn how you can model angiogenesis in the OrganoPlate, watch the on-demand webinar: "Modelling Angiogenesis From Start to Finish in the OrganoPlate®".
Dissatisfied With Your Angiogenesis Model? Read This

Dissatisfied With Your Angiogenesis Model? Read This
The article discusses the limitations of current in vitro angiogenesis models and highlights the OrganoPlate® as a more physiologically relevant, high-throughput platform for studying angiogenesis.

Dissatisfied With Your Angiogenesis Model? Read This
The article discusses the limitations of current in vitro angiogenesis models and highlights the OrganoPlate® as a more physiologically relevant, high-throughput platform for studying angiogenesis.

Dissatisfied With Your Angiogenesis Model? Read This
The article discusses the limitations of current in vitro angiogenesis models and highlights the OrganoPlate® as a more physiologically relevant, high-throughput platform for studying angiogenesis.

Dissatisfied With Your Angiogenesis Model? Read This
The article discusses the limitations of current in vitro angiogenesis models and highlights the OrganoPlate® as a more physiologically relevant, high-throughput platform for studying angiogenesis.

Dissatisfied With Your Angiogenesis Model? Read This
The article discusses the limitations of current in vitro angiogenesis models and highlights the OrganoPlate® as a more physiologically relevant, high-throughput platform for studying angiogenesis.

Dissatisfied With Your Angiogenesis Model? Read This
The article discusses the limitations of current in vitro angiogenesis models and highlights the OrganoPlate® as a more physiologically relevant, high-throughput platform for studying angiogenesis.

Dissatisfied With Your Angiogenesis Model? Read This
The article discusses the limitations of current in vitro angiogenesis models and highlights the OrganoPlate® as a more physiologically relevant, high-throughput platform for studying angiogenesis.

Dissatisfied With Your Angiogenesis Model? Read This
The article discusses the limitations of current in vitro angiogenesis models and highlights the OrganoPlate® as a more physiologically relevant, high-throughput platform for studying angiogenesis.

