Targeting HCC Tumor Microenvironment Interactions Using an Advanced HCC Patient-Derived On-Chip Model
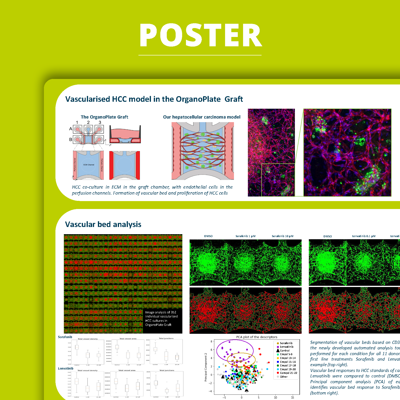
Hepatocellular carcinoma (HCC) is the most common primary liver cancer accounting for approximately 90% of cases and is strongly associated with chronic liver diseases such as cirrhosis. A biologically relevant, predictive, and user-friendly HCC model is required to expedite research and develop novel therapies.In this poster, we present the development of a screenable vascularized HCC model in the OrganoPlate® Graft using patient-derived material. This perfusable microvascular network is responsive to standard of care drugs and allows for phenotypic assessment of the vasculature organization. Here, we accomplished high-throughput screening of 30 treatment conditions, including
Targeting HCC Tumor Microenvironment Interactions Using an Advanced HCC Patient-Derived On-Chip Model

Targeting HCC Tumor Microenvironment Interactions Using an Advanced HCC Patient-Derived On-Chip Model
A vascularized, patient-derived HCC-on-a-chip model enabling high-throughput screening of drug responses and tumor–vasculature interactions—supporting phenotypic and cytokine analyses for advanced liver cancer research.

Targeting HCC Tumor Microenvironment Interactions Using an Advanced HCC Patient-Derived On-Chip Model
A vascularized, patient-derived HCC-on-a-chip model enabling high-throughput screening of drug responses and tumor–vasculature interactions—supporting phenotypic and cytokine analyses for advanced liver cancer research.

Targeting HCC Tumor Microenvironment Interactions Using an Advanced HCC Patient-Derived On-Chip Model
A vascularized, patient-derived HCC-on-a-chip model enabling high-throughput screening of drug responses and tumor–vasculature interactions—supporting phenotypic and cytokine analyses for advanced liver cancer research.

Targeting HCC Tumor Microenvironment Interactions Using an Advanced HCC Patient-Derived On-Chip Model
A vascularized, patient-derived HCC-on-a-chip model enabling high-throughput screening of drug responses and tumor–vasculature interactions—supporting phenotypic and cytokine analyses for advanced liver cancer research.

Targeting HCC Tumor Microenvironment Interactions Using an Advanced HCC Patient-Derived On-Chip Model
A vascularized, patient-derived HCC-on-a-chip model enabling high-throughput screening of drug responses and tumor–vasculature interactions—supporting phenotypic and cytokine analyses for advanced liver cancer research.

Targeting HCC Tumor Microenvironment Interactions Using an Advanced HCC Patient-Derived On-Chip Model
A vascularized, patient-derived HCC-on-a-chip model enabling high-throughput screening of drug responses and tumor–vasculature interactions—supporting phenotypic and cytokine analyses for advanced liver cancer research.

Targeting HCC Tumor Microenvironment Interactions Using an Advanced HCC Patient-Derived On-Chip Model
A vascularized, patient-derived HCC-on-a-chip model enabling high-throughput screening of drug responses and tumor–vasculature interactions—supporting phenotypic and cytokine analyses for advanced liver cancer research.

Targeting HCC Tumor Microenvironment Interactions Using an Advanced HCC Patient-Derived On-Chip Model
A vascularized, patient-derived HCC-on-a-chip model enabling high-throughput screening of drug responses and tumor–vasculature interactions—supporting phenotypic and cytokine analyses for advanced liver cancer research.

