The Beginner’s Guide to ECM Gels
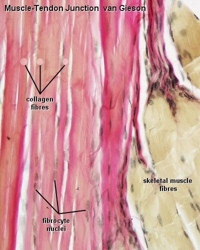
So, you’ve decided to give this 3D cell culture thing a spin. You’ve heard 3D culture can increase the physiological relevance of your culture and you’re excited to start! Immediately, you’ll run into a plethora of possible extracellular matrix (ECM) gels, raising many questions – How, exactly, do I bring my cells into the third dimension? Should I use a natural or artificial hydrogel? Which ECM is right for me?This article is a good place to start if you’d like to understand the ECM proteins that are used in existing protocols, or if you’d like to create or optimize a protocol of your own. Here, we’ll discuss the types of ECM proteins that exist in the body and some of the ECM hydrogels, both natural and synthetic, that are commonly used in 3D cell culture.No two organs are alike. Therefore, the optimal conditions for your culture depend on the organ or tissue you want to model. Be sure to read up on the ECM in your tissue of interest, as well as any 3D protocols published by other labs, to get a picture of the tissue microenvironment you’re aiming to recreate.ECM Proteins in the BodyThe extracellular matrix is a complex, hydrated networkpink fibrous collagen of proteins and polysaccharides that comprises much of the acellular mass in our bodies. Strong, stiff, stretchy, firm… different parts of our bodies use different ECM proteins to achieve different mechanical properties as also shown in this video. ECM proteins are also an important source of biochemical and biomechanical signals that support cell differentiation and function and can be as tissue-specific as cells themselves. Collagen is the most common protein in our bodies (about 1/3 dry weight) and quite strong. Much of it is fibrillar type I, II, and III collagen, which has excellent tensile strength due to intertwined, triple-helical structure. You can find fibrillar collagen predominantly in stress-bearing connective tissues such as tendons and ligaments, which are formed from many thick bundles of collagen fibers (dark pink) and very few cells (brown)1.Collagen fibers can also be found in less mechanically intense environments. In this image of the breast, pink fibrous collagen takes up a substantial amount of the volume in the stromal compartment2.Moreover, even very cell-dense tissues Liver Reticulin Stain like muscle, fat, and liver have small amounts of reticular collagen (black), composed of collagen III fibers, to help hold cells together3.By contrast, the nonfibrillar collagen IV does not form bundles but instead forms a mesh-like polymeric structure within the basement membrane. The basement membrane also referred to as the basal lamina, is a very thin, dense sheet of ECM protein that anchors and separates epithelial or endothelial tissue from stromal tissue. Each basement membrane is a complex, interconnected mix of multiple types of ECM proteins (containing at least one laminin, one or two nidogens, heparan sulfate proteoglycans, and type IV collagens). It has unique molecular compositions across tissues and plays an important role in a variety of processes, like blocking dissemination of metastatic carcinoma cells or filtration in the glomerulus of the kidney. Here, red collagen IV staining identifies basement membranes that separate the dermis (white) from the epidermis (blue) and blood vessels of the skin4.The ECM also contains many proteoglycans,embryonic chicken, stained with Alizarin Red and Alcian Blue to differentiate between hardened bone (in red) and the remaining cartilage model (in blue). composed of a protein core covalently linked to long chains of smaller sugar-decorated proteins called glycosaminoglycans. The long, linear sugar polymers provide the proteoglycans with a dense negative charge that attracts and retains water. Glycosaminoglycans include chondroitin sulfate, heparan sulfate, and hyaluronic acid (or hyaluronan). Chondroitin sulfate and heparan sulfate are particularly known for their capacity to sequester and display growth factors, either within the ECM itself or as membrane-bound ECM proteins on the cell surface. These ECM molecules form highly hydrated, loose networks and are expressed throughout the body, but especially in cartilage-like tissues. Cartilage, the translucent material covering your joints and providing shape to your ears and nose, is particularly rich in proteoglycans, as it needs a high water content to stay firm and slippery. Beautiful images of cartilage giving way to bone in developing embryos can be created using differential staining of glycosaminoglycans in cartilage (Alcian blue) and calcium in bone (Alizarin red)5.Elastin is another important ECM protein. Whileimage of an artery, elastin fibers are the curly purple lines interior and exterior to the wall collagen provides strength, elastin helps give stretchy tissues like lung, skin, and arteries a little stretchiness. Elastin is a long, hydrophobic protein that coils to minimize the exposure of its surface to water. When relaxed, elastin fibers are curly; when stretched, the fibers are forced to extend, providing physical resistance that rebounds when tension is relieved. In this image of an artery, elastin fibers are the curly purple lines interior and exterior to the wall6.ECM Proteins used in Cell CultureThe intricate spatial organization of different types of ECM in the body is, of course, organized by the cells themselves. It’s not easily replicated by laboratory researchers! We have to resort to extracting ECM from animal sources or using recombinant proteins from bioreactors, which may not fully recreate the in vivo niche. Fortunately for us, many cells are also capable of producing their own tissue-specific matrix in culture, and of remodeling various ECM proteins they encounter. An exception is posed by stem cells, which rely on ECM production by niche cells. You’ll find that embryonic stem cells require, for example, mouse embryonic feeder layers or a coating of ECM protein on their culture flasks7.We need to give cells a permissive environment for growth, differentiation, and function. Naturally occurring ECM proteins are an excellent place to start. ECM proteins that match those found in the body have some advantages over synthetic hydrogels. They contain sites for degradation by cell proteases, which permit cells to migrate and proliferate while embedded in the hydrogel. ECM proteins also natively contain peptide motifs that specifically bind cell surface adhesion proteins. Without these, non-malignant adherent cells will die.Fibrinangiogenesis assay in fibrin gelHow far back does 3D cell culture go? Embryologists in the 1920s developed organ culture techniques such as the watch glass method, which consisted of a concave glass surface holding a plasma clot in which the embryonic tissue fragment or organ rudiment was embedded8.A clot is a transitional ECM: fibrin gel covered in activated platelets located at an injury. Nowadays, the fibrin gel can be created in the lab from two components purified from plasma: the precursor protein, fibrinogen, and the enzyme thrombin, which cleaves fibrinogen into fibrin monomers that then self-assemble into a gel9. Conveniently, this reaction can occur under physiological conditions. The reaction may be tuned to alter the gelation time and mechanical properties of the resulting gel. This very soft (E<100 Pa) fibrous material is commonly used in 3D cell culture of endothelial cells, as fibrin alone can promote blood vessel formation10, and as a tissue sealant in clinical applications11. Below, a common angiogenesis assay in fibrin gel shows endothelial cells sprouting away from a bead12.On the other hand, since fibrin is only found in the body during clotting and wound healing, fibrin alone is not typically used in 3D cell culture for growing non-endothelial cells. In combination with other ECM proteins, it can make a 3D cell culture gel that is more prone to of angiogenesis.Collagen IIn the 1950s, researchers discovered how to extract and reconstitute collagen I derived from rat tail tendons into a 2D substrate and 3D gel for cell culture13. Currently, purified collagen I for 3D cell culture can be obtained from a variety of vendors, typically sourced from rat tail tendon or bovine skin or tendon. The acid-solubilized collagen I protein solution is usually neutralized on ice and might solidify when warmed. Some formulations do not require a separate neutralization step. They are stiffer than fibrin and can be adjusted by tuning collagen concentration and crosslinking, with a range of elastic moduli from around 100 Pa to over 1 kPa14.Like fibrin, collagen I matrices are fibrous, though the initial gels lack some of the larger-scale fiber structure found in tissues. In the images on the right15, self-assembled collagen I gel in vitro (a) shows shorter fiber lengths than mouse dermis (b), mouse bone (c), and human ovary (d).The difference? Cells such as fibroblasts are responsible for aligning and bundling collagen in the body, whereas the collagen that has been disassociated from animal sources has lost this large-scale structure. This process can be recreated in the lab – contractile cells embedded in collagen gels in 3D culture can align and deform collagen fibers around them. These nano-structured collagen fibers can not only increase the local stiffness of the tissue but also act as conduits to support directed cell migration and invasion both in vitro and in vivo16.Basement membrane extractBasement membrane extract was first isolated in 1977 from mouse chondrosarcoma Engelbreth-Holm-Swarm tumors. Now it is more commonly known by its many commercial names (for example, Matrigel, Cultrex, ECMatrix, and Geltrex). It is highly complex and contains a variety of solubilized basement membrane proteins, primarily laminin, collagen IV, entactin/nidogen, and heparan sulfate proteoglycan. Growth factors like EGF, IGF1, TGFβ, PDGF, and NGF have also been detected. Nevertheless, the basement membrane can be purchased in a growth factor reduced form17 and as an extract derived only from human cell culture18. Basement membrane extract is liquid at low temperatures and polymerizes when warmed to a gel with an elastic modulus of only a couple hundred Pa, depending on protein concentration19.Unlike collagen I gels, basement membrane extract does not readily form fibrils or long-range structures. (This may relate to the observation that angiogenic sprouting of endothelial cells is reduced when embedded in Matrigel alone compared to fibrin or collagen.)Basement membrane ECM can support many biological processes, from stem cell maintenance20 to cell polarity21. It is very often used to support 3D cell culture and organoid growth. On the right, researchers looking at branching morphogenesis in mouse mammary epithelial organoids discovered that the ratio of Matrigel (Mtg, M) to collagen I (Col, C) in their ECM could affect the morphology and cell sorting of luminal and basal epithelial cells22.Branching morphogenesis in mouse mammary epithelial organoids Unfortunately, the complexity of basement membrane extract lends itself to high lot variability and makes it challenging to identify the individual factors responsible for its desirable properties. It is not uncommon for a lab to buy an entire lot of Matrigel at once to reduce variability between experiments!Other ECM ProteinsThese proteins don’t usually make stable hydrogels on their own, but can be doped into a larger ECM matrix, chemically modified to form a 3D gel, or used for coating 2D cell culture substrates for improved adhesion, especially for stem cells. Purified, especially recombinant, proteins aren’t cheap to add to a 3D cell culture system, which might be why they aren’t as ubiquitous in the literature as plain collagen I or Matrigel.LamininLaminin, the main component of the basement membrane (along with collagen IV) is a globular heterotrimeric protein with binding sites for many other ECM proteins and cells. Purified exogenous laminin has been used to demonstrate a specific role for laminin in maintaining epithelial cell polarity in 2D and 3D23. It can be purified from human cells in culture or mouse Engelbreth-Holm-Swarm tumors.FibronectinFibronectin, like fibrin, is a component of blood clots and the wound healing microenvironment but is also secreted by mesenchymal cells as an insoluble component of the ECM. It has several functional domains capable of binding other fibronectin molecules (forming fibrils), other ECM proteins such as collagen and fibrin, and a very wide range of cell-matrix adhesion molecules (integrins)24. Fibronectin is typically isolated from human or bovine plasma or derived from recombinant expression.Tropoelastin or ElastinTropoelastin is the soluble precursor of elastin and may be included in biomaterials in which elasticity and stretch is a desirable feature (for example, artificial skin constructs)25,26. It is typically purified from human or animal skin, aorta, and lung, or produced by recombinant expression in bacteria.Hyaluronic Acid (Hyaluronan)Modified hyaluronic acid can be used as a hydrogel for 3D cell culture. It is ubiquitous throughout the body and has a long history of clinical use. It can be degraded in the body by hyaluronidase. However, as a glycosaminoglycan with anti-adhesive properties in vitro, it needs to be combined with other ECM proteins or chemically modified to support cell attachment. These semi-synthetic hyaluronic acid hydrogels can be made in-house or are commercially available under the name HyStem. Hyaluronic acid can be chemically synthesized or derived from recombinant expression in bacteria.Chondroitin SulfateChondroitin sulfate, like hyaluronic acid, is a glycosaminoglycan that can be chemically modified to support hydrogel formation and cell attachment. It is commonly found in cartilage, where it provides resistance to compression due to the negative charge repulsion of its sulfate groups.And More…There’s a world of less-common ECM components that can be explored for use in 3D cell culture, such as osteopontin, vitronectin, nidogen, and so on. You might not even need to add them in yourself – functional, differentiated cells commonly secrete tissue-specific ECM proteins. For example, osteopontin, elastin, and chondroitin sulfate are produced by cells in bone, artery/skin, and cartilage. It’s up to the individual research team to determine which can be beneficial for their particular cell system, but hopefully, the basic ECM components listed here can be a good starting point.Artificial HydrogelsThe sky’s the limit for artificial hydrogels. With the right bioconjugation techniques, you can customize designer hydrogels to your heart's content. The most notable draw of artificial hydrogels for 3D cell culture is the excellent level of control researchers have over their material and biological properties. Artificial hydrogels are a blank slate upon which cell-matrix interactions can be studied. So blank that they are generally unable to support cell function unless you chemically modify them.Artificial hydrogels come in many forms, but at their most basic, they are composed of a polymer backbone, usually functionalized with adhesion ligands to support cell attachment.Polymer BackbonesNaturally-Derived PolymersNaturally, derived polymers can be ECM components found in our bodies, such as the aforementioned hyaluronic acid and chondroitin sulfate, but researchers also make use of polysaccharides produced in other organisms as convenient building blocks for hydrogels.Alginate (alginic acid) - Alginate is a linear, negatively charged polysaccharide derived from red algae that can be crosslinked in a variety of ways. Ionic crosslinking (using Ca2+ and Ba2+) occurs in seconds, depending on the concentration and solubility of your cationic salt. The ionic intermolecular interactions can be broken and reformed under strain, allowing plastic deformation. The crosslinks steadily degrade through diffusion of ions under physiological conditions. Covalent crosslinking can create a permanent hydrogel. Chitosan - Chitosan is derived from the chitin exoskeletons of shellfish. It is a linear polysaccharide, similar to glycosaminoglycans, but bearing a net positive charge in physiological conditions due to its amino groups. Gelation can be triggered by adjusting the pH or by adding multivalent anions. Dextran - Dextrans are branched polysaccharides of varying lengths and levels of branching that are produced by bacteria. They can be chemically modified to support crosslinking and have been used clinically27. Agarose - Agarose is the main component of agar and is derived from red algae. It is a linear polysaccharide that dissolves upon heating and gelates when cooled, as intermolecular hydrogen bonds form. Agarose can form gels between 1 to ~1000 kPa in elastic modulus based on molecular weight and concentration.Synthetic PolymersThere is a variety of synthetic polymers commonly used to create hydrogels for cell culture. They are typically bio-inert and biocompatible (i.e., not overtly toxic to cells), and contain functional groups that can be easily modified to adjust gelation properties, adhesion, and biological activity.Polyethylene glycol (PEG) - Available in a variety of chain lengths and branching densities, PEG hydrogels are common synthetic polymers used in 3D cell culture.Poly(acrylamide) (PAAm) - PAAm hydrogels have been used frequently in mechanotransduction studies, due to their tunable, linear elasticity (from ~kPa to ~MPa).Poly(lactic acid), poly(lactic-co-glycolic acid), and poly(caprolacton) (PLA, PLGA, PCL) - These polymers share a common property: they are hydrolytically degradable. They are frequently incorporated in co-polymers (e.g., with PEG) to improve their degradability. They are used in biomaterials that are meant to be resorbed in the body over time, such as sutures. In tissue engineering, degradable ECM could be replaced by new tissue. Precisely tuning the degradation rate to match that of tissue formation and accounting for the negative effects of acidic degradation products is a key consideration in using these materials for cell culturePoly(vinylalcohol) (PVA) - A simple polymer that contains many convenient hydroxyl groups for functionalization.Poly(NIPAAm) - PNIPAAm (and copolymer PNIPAAm-PAAc) is best known for its thermoresponsive, reversible sol-gel transition near physiological temperatures. Cells grown and adhered to solid PNIPAAm can thus be released simply by lowering the temperature.Designer PeptidesAlas, it would be impossible to go into the details of a class of hydrogels that can vary so tremendously. Suffice it to say that various researchers have generated recombinant peptides that assemble into hydrogels under certain conditions, typically by adjusting the pH, adding an enzyme, photopolymerization, or combining precursors. Elastin-like peptides, for example, are specifically engineered to recapitulate the high extensibility of elastin by mimicking its pentapeptide repeat motifs28. Peptide-based hydrogels are commercially available, such as PuraMatrix29a and Biogelx29b, but their precise structures are generally proprietary.Chemical Modification of Artificial HydrogelsNow, this is the important part, if you ask me! The polymer backbone isn’t quite as important as what’s attached to it.Polymerization and MechanicsThese polymer backbones need crosslinking to form stable hydrogels. The degree and type of crosslinking can also influence the material’s physical properties, such as stiffness and creep (i.e. deformation over time).Photopolymerization can be spatially controlled using a mask or laser. Vinyl and acrylate groups are UV photopolymerizable with the appropriate radical initiator and crosslinker. However, if polymerization must occur while cells are embedded within hydrogel, the potential negative effects of UV irradiation and radical polymerization on the health of the cells should be taken into account.Several enzyme-mediated crosslinking reactions can be used to crosslink functionalized polymers. Substrate-specific enzymes that operate at physiological pH and temperature are often considered safer for cells than photopolymerization or reactions involving toxic reagents or byproducts. Some examples of such enzymes include horseradish peroxidase or hematin (with hydrogen peroxide), laccase, and tyrosinase for phenol-functionalized hydrogels30; transglutaminase, phosphopantetheinyl transferase, lysyl oxidase, and plasma amine oxidase31.Cell AttachmentAn obvious approach to promoting cell attachment is to incorporate actual ECM proteins into an artificial hydrogel, either by physically mixing the gels into an interpenetrating network or by covalently conjugating the hydrogel and ECM protein using your favorite bioconjugation technique.However, for those seeking highly specific control over their hydrogel’s adhesive properties, cell-adhesive amino acid sequences can be attached to the gel. The key example of an adhesion site is the RGD amino acid sequence, first identified in fibronectin. RGD is by far the most common cell adhesion motif used in artificial hydrogels, though others, such as FHRRIKA, GFOGER, YGISR, and KRSR have been identified as integrin-binding sites in natural ECM proteins. Minimal amino acid sequences may, in the full-length protein, be complemented by neighboring domains to alter binding strength or integrin specificity. Like any peptide, these motifs can be covalently conjugated (e.g., via EDC-NHS or sulfo-SANPAH) to allow cell attachment to artificial hydrogels32.BiomoleculesBiomolecules, including but not limited to growth factors and gene therapies, can be presented to cells in 3D culture by direct encapsulation in the hydrogel, freely diffusing through its pores, and/or interacting electrostatically with the polymer itself (A). To prevent losses from diffusion, they may instead be encapsulated or bound to embedded micro/nanoparticles33,34 (C). These particles can be designed to degrade or allow diffusion over time for a low, sustained dose, or stay intact for high, spatially restricted doses. Sustained delivery can also be achieved by covalently linking the cargo to the polymer using a degradable linker that can be released by UV, hydrolysis, or cell protease activity (B). Protease-specific peptides have been characterized and are commercially available35 . Note that direct, covalent conjugation of a biomolecule may alter its signaling function relative to its soluble or electrostatically bound form36. In the image37 below you’ll find an overview of schematic structures of PEG hydrogels formed via: A chain-growth, B step-growth, and C mixed-mode step and chain growth polymerization.chematic structures of PEG hydrogels formed via: A chain-growth, B step-growth, and C mixed-mode step and chain growth polymerization.DegradationIf your gel is not degradable, cell motility and growth will be constrained by its pore size and sinuosity. ECM proteins found in the body will generally be susceptible to degradation by cell proteases, but artificial ECMs require specific design. As noted above, there are several peptide motifs targeted by specific proteases. These linkers can be used to make protease-cleavable crosslinks in the structure of your hydrogel.Hydrogels can also be made susceptible to hydrolysis, either by including hydrolysable co-polymer blocks (PLA, PLGA, PCL) in the backbone, or using ester bonds, which can be hydrolyzed in physiological conditions.Photodegradable hydrogels are another interesting strategy, with the opportunity to make precise structural changes to the hydrogel even as cells are still growing within it. Benzyl ether bonds in crosslinks or part of the polymer backbone can be degraded by UV light, and have been used to create photodegradable hydrogels for 3D cell culture. In the image below you'll find a schematic overview of this strategy38.Strategy to create photodegradable hydrogels for 3D cell cultureBest Practices for Using ECM HydrogelsYou will find that these viscous solutions don’t pipette as nicely as a medium. They pick up bubbles that will never pop, but remain inside the gelating hydrogel, ruining imaging and cell dispersal. How frustrating! I can’t offer you the hands-on experience that will ultimately solve your handling problems, but here are a few tips for working with ECM gels based on my experiences with collagen, Matrigel, and alginate. If you’re using a different (especially synthetic) system, be sure to adjust this advice based on your gelation protocol.Briefly put, the characteristics that make a good assassin will also make your hydrogels much better: Cold, fast, and deliberate.ColdECM gels such as collagen, basement membrane ECM, and fibrin are temperature-sensitive. Keep your aliquots frozen at -80°C (or package instructions), and thaw slowly and evenly by immersing in a wet ice bath. For large volumes, you may even need to thaw overnight. Once thawed, keep them and anything they touch ice-cold. That includes pipette tips, tubes, and plates. A metal tube block on ice may be helpful here. Do not refreeze.FastMorphology in epithelial organoidsThis will vary based on your hydrogel type and concentration, but even after a few minutes or seconds in the right conditions (e.g., the addition of thrombin or neutralization buffer), the ECM solution will no longer be workable. Even if the macroscopic appearance of the solution does not change, other biologically relevant changes could be occurring under your nose! For example, collagen fibers that have been neutralized will spontaneously self-assemble into thicker fibers even when on ice, before the cross-linking step that leads to gelation. This can dramatically affect cell morphology, as demonstrated by these epithelial organoids39.DeliberateMove quickly but not sloppily. Bubbles, once introduced, are extremely hard to get out of a viscous solution. Gel solutions will enter and leave a pipette tip slowly; don’t rush or you may use the wrong volume or create bubbles. If using a syringe, draw up the solution, then invert the syringe, so bubbles float to the opening. Gently tap the sides and rotate the plunger to dislodge bubbles, then depress the plunger to expel them. Diffusion will also be slowed, so when pipetting up and down to distribute cells, move the tip to different parts of the solution to ensure thorough mixing. If your cell suspension has a different color than the gel (e.g., from phenol red), ensure that the color is uniform throughout after mixing.Have fun!Ultimately, 3D cell culture is about Trietsch et al. Membrane-free culture and real-time barrier integrity assessment of perfused intestinal epithelium tubescreating a microenvironment for cells that provides in vivo-like cues for proper differentiation and phenotype. ECM hydrogels are an important part of that environment. Though there’s a dazzling array of options, don’t worry – often a simple solution works well for the specific questions you wish to ask. As you develop and prototype your assays, be sure to measure the readouts that are most relevant to you.A good example of the importance of an extracellular matrix mimic, such as a collagen gel is given in the publication of Trietsch et al. in Nature Communications. In the paper, perfused gut tubes are grown in the OrganoPlate® against a patterned collagen gel (see left figure). Increased expression of proteins like MRP2, Erb2, and Glut2 was found against gel with respect to those parts of the tube that were not against the collagen (see right figure).In the OrganoPlate, you can use a range of ECM gels that can transition from a liquid to a solid state. These gels include but are not limited to collagen-I, Matrigel®, Matrigel®-growth factor reduced, BME, and HyStem gels. Depending on your plate and gel of choice, you need 1-2 µL of gel per chip.Happy optimizing, and best of luck in your scientific adventures!
The Beginner’s Guide to ECM Gels

The Beginner’s Guide to ECM Gels
This guide reviews ECM options for 3D cell culture—covering natural proteins (collagen, fibrin, Matrigel®, hyaluronic acid) and synthetic hydrogels (PEG, alginate, designer peptides)—and offers practical tips for selecting and handling matrices to best mimic in vivo microenvironments.

The Beginner’s Guide to ECM Gels
This guide reviews ECM options for 3D cell culture—covering natural proteins (collagen, fibrin, Matrigel®, hyaluronic acid) and synthetic hydrogels (PEG, alginate, designer peptides)—and offers practical tips for selecting and handling matrices to best mimic in vivo microenvironments.

The Beginner’s Guide to ECM Gels
This guide reviews ECM options for 3D cell culture—covering natural proteins (collagen, fibrin, Matrigel®, hyaluronic acid) and synthetic hydrogels (PEG, alginate, designer peptides)—and offers practical tips for selecting and handling matrices to best mimic in vivo microenvironments.

The Beginner’s Guide to ECM Gels
This guide reviews ECM options for 3D cell culture—covering natural proteins (collagen, fibrin, Matrigel®, hyaluronic acid) and synthetic hydrogels (PEG, alginate, designer peptides)—and offers practical tips for selecting and handling matrices to best mimic in vivo microenvironments.

The Beginner’s Guide to ECM Gels
This guide reviews ECM options for 3D cell culture—covering natural proteins (collagen, fibrin, Matrigel®, hyaluronic acid) and synthetic hydrogels (PEG, alginate, designer peptides)—and offers practical tips for selecting and handling matrices to best mimic in vivo microenvironments.

The Beginner’s Guide to ECM Gels
This guide reviews ECM options for 3D cell culture—covering natural proteins (collagen, fibrin, Matrigel®, hyaluronic acid) and synthetic hydrogels (PEG, alginate, designer peptides)—and offers practical tips for selecting and handling matrices to best mimic in vivo microenvironments.

The Beginner’s Guide to ECM Gels
This guide reviews ECM options for 3D cell culture—covering natural proteins (collagen, fibrin, Matrigel®, hyaluronic acid) and synthetic hydrogels (PEG, alginate, designer peptides)—and offers practical tips for selecting and handling matrices to best mimic in vivo microenvironments.

The Beginner’s Guide to ECM Gels
This guide reviews ECM options for 3D cell culture—covering natural proteins (collagen, fibrin, Matrigel®, hyaluronic acid) and synthetic hydrogels (PEG, alginate, designer peptides)—and offers practical tips for selecting and handling matrices to best mimic in vivo microenvironments.

