Oegstgeest, August 22, 2025 – A collaborative study between MIMETAS and Roche Pharma Research and Early Development (pRED) has identified key mechanisms by which the ApoE4 genetic variant—a major risk factor for Alzheimer’s disease—influences molecular transport processes in the brain.
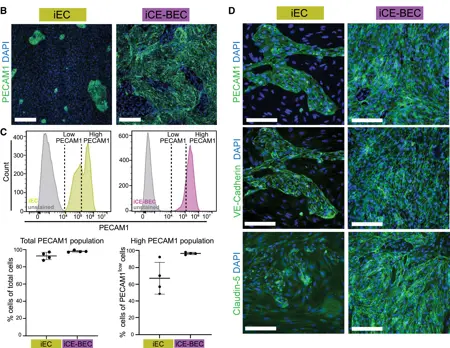 A key aspect of this research was the development of a new iPSC-differentiation protocol to generate brain endothelial cells (iCE-BECs). These cells closely mimic the blood–brain barrier (BBB), showing strong barrier properties, expression of brain-specific markers without epithelial ones, and supporting receptor-mediated transcytosis. This advancement makes them particularly well-suited for investigating how genetic risk factors affect brain endothelial cell function.
A key aspect of this research was the development of a new iPSC-differentiation protocol to generate brain endothelial cells (iCE-BECs). These cells closely mimic the blood–brain barrier (BBB), showing strong barrier properties, expression of brain-specific markers without epithelial ones, and supporting receptor-mediated transcytosis. This advancement makes them particularly well-suited for investigating how genetic risk factors affect brain endothelial cell function.
The study further leveraged Roche’s BrainShuttle technology, enabling precise evaluation of molecular transport across the BBB. The combined expertise allowed the research team to not only model disease-relevant mechanisms, but also explore potential therapeutic delivery pathways.
Using iPSC lines engineered to carry either the ApoE3 or ApoE4 genotype, the researchers discovered that ApoE4 alters the structure and function of early endosomes—key components of the intracellular transport system. Specifically, ApoE4 was associated with enlarged, less acidic endosomes and reduced sorting capacity. In addition, ApoE4 cells exhibited reduced intracellular iron levels, which triggered upregulation of the transferrin receptor (TfR1). Despite this increase, the rate of transferrin transport remained unchanged, indicating a disconnect between receptor expression and functional transport.
Together, these findings highlight MIMETAS’ contribution in advancing physiologically relevant human BBB models and Roche’s technology in enabling mechanistic studies of transcytosis. The study provides new insights into the cell-autonomous effects of ApoE4 at the BBB and emphasizes how genetic risk factors can subtly alter brain endothelial function—potentially influencing therapeutic delivery to the brain.
