New Study on 3D Human Artery Model with Unidirectional Flow
Oegstgeest, April 22, 2025 – MIMETAS published a new peer-reviewed study detailing a significant advancement in vascular disease modeling. The paper, titled “Microfluidic artery-on-a-chip model with unidirectional gravity-driven flow for high-throughput applications”, introduces a novel in vitro model that replicates the physiological and pathological behavior of human arteries.
Bridging the Gap in Cardiovascular Research
Cardiovascular diseases remain the leading cause of death worldwide, yet drug development in this field has been hindered by poor translatability of preclinical models. The newly developed model by MIMETAS directly addresses this bottleneck, developed using their newly launched Uniflow™ Technology that supports unidirectional, gravity-driven fluid flow to more accurately simulate blood flow conditions.
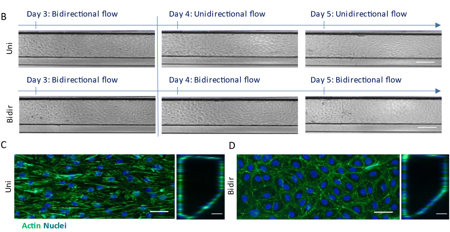
Under unidirectional flow, human coronary artery endothelial and smooth muscle cells formed more physiologically relevant vessel structures that exhibit vascular relaxation. Key indicators such as endothelial alignment, fibronectin deposition, and inflammatory markers like ICAM-1 and IL-6 were assessed. The model also responded predictably to inflammatory cytokines, providing a reliable platform for studying early-stage atherosclerosis and vascular inflammation.
Technological Innovation with Real-World Impact
Unlike traditional pump-based microfluidic systems, MIMETAS’ Uniflow technology leverages an interval rocking mechanism and capillary barriers to maintain controlled, one-way fluid movement. This innovation simplifies operation and improves scalability without compromising physiological relevance. Additionally, the platform's compatibility with lab automation systems positions it as a practical tool for high-throughput drug screening.
Notably, this technology allows researchers to distinguish between healthy and diseased states of vasculature. Vessels exposed to unidirectional flow displayed hallmarks of vascular health, including reduced fibronectin secretion and maintenance of a non-contractile smooth muscle phenotype. In contrast, bidirectional flow conditions mimicked early signs of endothelial dysfunction, such as increased fibronectin and lipid accumulation. However, both unidirectional and bidirectional models demonstrated responses to inflammatory triggers, reflecting their physiological relevance.
UniFlow technology is available exclusively available through MIMETAS’ service portfolio and partnerships. It is currently implemented in a broad range of their human 3D tissue models—including liver, lung, cancer, and vascular assays—to study immune, cardiovascular, metabolic, infectious, CNS, and oncological diseases, as well as ADME/Tox applications.
Expanding the Frontier of Organ-on-a-Chip Models
The co-culture approach—combining endothelial and smooth muscle cells—further strengthens the physiological relevance of the model. The inclusion of inflammatory stimuli such as TNFα and IL-1β demonstrated the model’s capacity to emulate acute vascular inflammation and capture the dynamic response of endothelial and smooth muscle cells.
“This publication marks a huge step toward scalable, human-relevant models for cardiovascular research,” said Dr. Lenie van den Broek, co-author of the study and Director of Biology Discovery at MIMETAS. “Our findings demonstrate the platform’s robustness in replicating arterial biology and its utility for therapeutic development.” With this artery-on-a-chip model, MIMETAS aims to accelerate innovation in cardiovascular drug development and deepen scientific understanding of vascular pathologies.
