Leiden, September 30, 2022 – Scientists from MIMETAS develop a multiplexed assay to quantify reactive oxidative species (ROS) and cell viability in organ-on-a-chip models. This article has just been published in the Journal of Redox Biology.
Reactive oxygen species (ROS) are natural by-products of cellular oxidative metabolism. At low levels, ROS plays a key role in the processes such as regulation of cell proliferation, cell death, differentiaion, vasular tone, as well as angiogenesis. However, at high concentrations, ROS are harmful molecules that oxidize macromolecules, causing structural damage to cells and eventually apoptosis. This process is called oxidative stress (OS) and is not completely understood. Intracellular accumulation of ROS contributes and is implicated in many detrimental conditions including endothelial dysfunction, atherosclerosis, aging, and cancer. To better understand the role of OS and ROS in physiological and pathophysiological processes, quantifying ROS is of utmost importance.
Unfortunately, the quantification of ROS in vivo is challenging and is limited to in vitro studies due to its highly reactive nature, short half-life, and localization in discrete sub-cellular compartments. Overcoming these challenges warrants physiologically relevant in vitro models, which are currently lacking. The use of more advanced in vitro cell culture techniques such as 3D cell cultures or Organ-on-a-Chip, rather than simple 2D models would enable better recapitulation of the complexity and physiology typical of the in vivo situation.
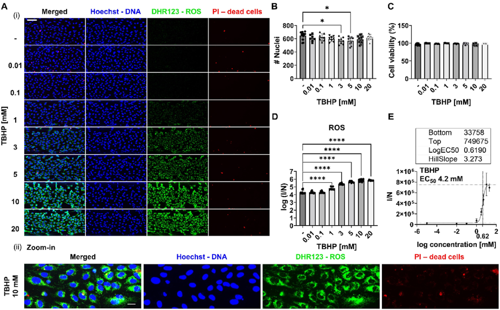 In this publication, the authors describe the development, validation and application of a scalable multiplexed versatile live-cell and image-based assay to quantify ROS accumulation or depletion together with cell viability in 3D organ-on-a-chip in vitro models developed in the OrganoPlate®. Live cells were stained for ROS, dead cells, and DNA and confocal images were analyzed to quantify ROS probes and determine the number of nuclei and dead cells. Interestingly, the results showed that on-a-chip models are more prone to scavenge ROS rather than accumulate them, which is in line with previous studies where they compared OS profile in on-a-chip and 2D cell culture models. Moreover, the assay demonstrated high sensitivity, distinguishing between different phenotypes of endothelial cells based on the level of OS to detect higher level in tumor than normal cells. Taken together, this assay is a valuable tool for envisioned to enable the unravelling of mechanisms behind OS and ROS accumulation.
In this publication, the authors describe the development, validation and application of a scalable multiplexed versatile live-cell and image-based assay to quantify ROS accumulation or depletion together with cell viability in 3D organ-on-a-chip in vitro models developed in the OrganoPlate®. Live cells were stained for ROS, dead cells, and DNA and confocal images were analyzed to quantify ROS probes and determine the number of nuclei and dead cells. Interestingly, the results showed that on-a-chip models are more prone to scavenge ROS rather than accumulate them, which is in line with previous studies where they compared OS profile in on-a-chip and 2D cell culture models. Moreover, the assay demonstrated high sensitivity, distinguishing between different phenotypes of endothelial cells based on the level of OS to detect higher level in tumor than normal cells. Taken together, this assay is a valuable tool for envisioned to enable the unravelling of mechanisms behind OS and ROS accumulation.
