Modelling and Prevention of Acute Kidney Injury
Leiden, February 24, 2022 – Scientists from MIMETAS and Astellas introduce a combined human renal proximal tubule/blood vessel-on-a-chip model in the OrganoPlate® 3-lane 40 to study acute kidney injury (AKI) caused by renal ischemia/reperfusion injury (rIRI). This article has just been published in Kidney360.
The kidneys are responsible for removing waste products from our blood, while simultaneously facilitating the reuptake of essential nutrients. This results in a high energy demand, and dependency on supply of oxygen and nutrients through the blood flow. Both disruption (ischemia) and the restart (reperfusion) of the blood flow can cause a loss of function of kidney cells and ultimately lead to AKI. This results in over 13 million yearly diagnoses, where a person’s kidneys suddenly shut down, causing toxins to accumulate in the bloodstream. AKI progression can lead to chronic kidney injury, cardiovascular pathology, and ultimately death.
To study the systemic symptoms of AKI, in vivo animal models are often used. Unfortunately, due to interspecies differences, not all drug candidates that shown efficacy in the animal models translate to efficacious candidates in clinical trials. Similarly, traditional 2D in vitro models fail to recapitulate the kidney structure and function, which makes them unsuited to predict clinical outcomes. Organ-on-a-chip technology offers a physiologically relevant alternative by culturing in vitro models in 3D to mimic the structure and function of the kidney, increasing the predictive value required for drug development and disease research.
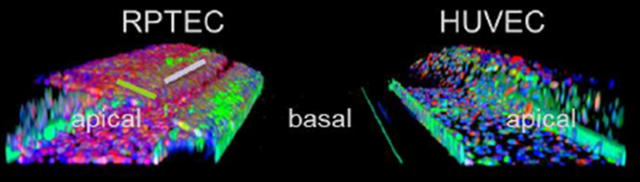
In this publication, an advanced kidney model was developed to study rIRI induced AKI. This model is based on a perfused 3D proximal kidney tubule in addition to a 3D perfused blood vessel, cultured from RPTEC and HUVEC cells, respectively. In this model, rIRI could be captured by altering oxygen levels, nutrient availability, and perfusion flow. It was shown that a combination of low oxygen, reduced glucose, and interrupted flow was potent to significantly damage the kidney tubules. Additionally, the damaging effect was amplified upon reperfusion of the tubules and could be alleviated through an adenosine treatment. The robustness and high-throughput capabilities of the model make it a valuable asset to advance pathophysiological research and drug development against AKI.
